Mercury: The Cause of Congenital Minamata Disease
Humans can be exposed to various forms of mercury. Elemental mercury, as pictured on the left, has a low vapor pressure. This allows it to transition into a gas very easily allowing for absorption through inhalation (Suzuki et al. 1991).
Inorganic and organic mercury can also be absorbed through the lungs, as well as through the skin and digestive system (Suzuki et al. 1991).
As a consequence of industrial waste and pollution, mercury can be released into the environment. Thus, mercury collects in the tissues of the exposed organisms which can include fish and other seafood that are caught and sold as food (Gochfeld 2003).
Inorganic and organic mercury can also be absorbed through the lungs, as well as through the skin and digestive system (Suzuki et al. 1991).
As a consequence of industrial waste and pollution, mercury can be released into the environment. Thus, mercury collects in the tissues of the exposed organisms which can include fish and other seafood that are caught and sold as food (Gochfeld 2003).
How You Are Exposed
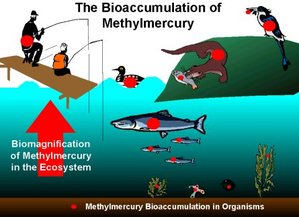
The image (Gochfeld 2003) to the right shows how mercury accumulates through the food chain that includes humans.
Another major source of mercury are dental fillings that can be made up of up to 50% mercury (Lorscheider et. al). Research shows that the mercury in fillings can be released over time and absorbed through the lungs and stomach (Lorscheuder et. al).
Occupational exposure such as working with dental fillings and other products that contain mercury is another way mercury can get into your system (Gochfeld 2003).
Another major source of mercury are dental fillings that can be made up of up to 50% mercury (Lorscheider et. al). Research shows that the mercury in fillings can be released over time and absorbed through the lungs and stomach (Lorscheuder et. al).
Occupational exposure such as working with dental fillings and other products that contain mercury is another way mercury can get into your system (Gochfeld 2003).
From Mom to Baby

Congenital Minamata Disease occurs as a result of prenatal exposure to mercury (Suzuki et. al 1991). While the developing fetus is protected from physical exposure in the mother's womb, the fetus is dependent on its mother for nutrition and oxygen through the placenta. While the fetal blood and maternal blood do not mix, transported molecules are exchanged at the placenta through diffusion and active transport (Kajiwara).
After exposure to mercury, the maternal blood will exhibit some presence of the metal. While the placenta typically acts as a defensive barrier to toxins, mercury is transported into the fetal blood through transporters that normally bring in neutral amino acids that are important for fetal growth and development (Suzuki et al. 1971)(Kajiwara). Research has shown that this system causes the fetal blood to have a higher concentration of mercury than the maternal blood (Suzuki et al. 1971). Once in the fetal blood, mercury can easily pass through the blood-brain barrier - exposing the developing fetal nervous system to the neurotoxic metal (Spyker 1972).
After exposure to mercury, the maternal blood will exhibit some presence of the metal. While the placenta typically acts as a defensive barrier to toxins, mercury is transported into the fetal blood through transporters that normally bring in neutral amino acids that are important for fetal growth and development (Suzuki et al. 1971)(Kajiwara). Research has shown that this system causes the fetal blood to have a higher concentration of mercury than the maternal blood (Suzuki et al. 1971). Once in the fetal blood, mercury can easily pass through the blood-brain barrier - exposing the developing fetal nervous system to the neurotoxic metal (Spyker 1972).
Mercury and the Developing Nervous System
Microtubules
Mercury has widespread effects on the developing nervous system. However, its effect on microtubules is a major contributor to the qualities that characterize Congenital Minamata Disease (Castoldi 2001).
Tubulin subunits, that make up microtubules, are essential cytoskeleton components for neurons especially during development when axons and their growth cones need to reach specific targets (Alberts 2008).
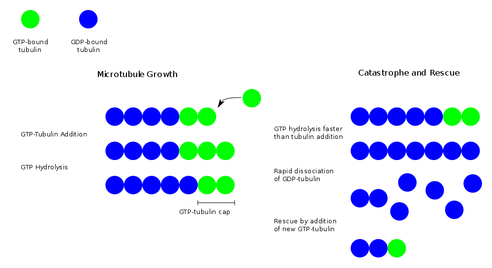
In order to build onto tubulin to elongate a microtubule, free tubulin molecules must be bound to GTP. Once bound to GTP, the free tubulin attaches onto the tubulin that is already part of the microtubule and the microtubule is further polymerized (Alberts 2008). However at the same time, the GTP-tubulin that is now part of the microtubule is converted to GDP-tubulin by an enzyme. If the rate of adding GTP-tubulin is faster than the conversion to GDP-tubulin, the microtubule will continue to grow (Alberts 2008). If the addition of GTP-tubulin were to be stopped or slowed, and the GTP-tubulin at the leading end of the microtubule were to be converted to GDP-tubulin then a cellular event called "catastrophe" would occur. During catastrophe, a microtubule without GTP-tubulin on its leading end will quickly depolymerize or shorten by freeing the tubulin subunits. This process or shortening can be halted when a GTP-tubulin binds to the depolymerizing microtubule (Alberts 2008). The following image (Sigel & Sigel 2008) summarizes this process.
During development, neurons, growth cones and axons require a lot of activity from their cytoskeleton to move them toward their appropriate targets. However, when they are exposed to mercury, the mercury competes with GTP for the binding site on tubulin (Leong 2001). Thus, if tubulin cannot bind to GTP, then all tubulin in a microtubule becomes converted to GDP-tubulin and the microtuble depolymerizes rapidly (Alberts 2008). While normal microtuble depolymerization does occur in normal cells, the rapid depolymerization of microtubles caused by mercury causes neurofibril structures to be left behind (Leong 2001). Without the structural support of the microtubles, the neurofibrils end up forming aggregates in the neural tissue (Leong 2001). The following video from the University of Calgary (Lorscheider 2001) provides a helpful visualization of this process and mercury's effects.
The degenerative effects of mercury on neuronal microtubules affect neuron body and axon motility. These actions are imperative for specific neural developmental processes such as creating appropriate neural connections as well as the appropriate formation of the cortical cortex layers (Suzuki et al. 1991). Axon growth is necessary for neurons to communicate with neurons in other areas of the nervous system, and neuronal cell body movement is needed to achieve the inside-out patterning of the cortex (Suzuki et al. 1991).
The Cerebellum
While mercury's effect on the nervous system is widespread, various research studies have pinpointed the cerebellum as an area that is especially affected for a multitude of reasons (Suzuki et al. 1991):
Inhibited Transcription
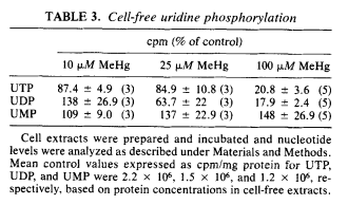
One of the effects of exposure to mercury, is the disruption of cerebellar protein synthesis (Sarafian 1986). In order for RNA polymerases to produce the appropriate mRNA, the necessary nucleotide triphosphates need to be present. In order for the RNA polymerase to transcribe the appropriate mRNA, uridine triphosphate (UTP) needs to be present in order to form uracil, an RNA nucleobase (Alberts 2008). The formation of uridine triphosphate for the use of the RNA polymerase is derived from the action of phosphorylating enzymes on uridine monophosphate (UMP). The enzyme uridine monophosphate kinase converts UMP to uridine diphosphate (UDP) and the enzyme uridine diphosphate kinase converts UDP to UTP (Alberts 2008). The following table (Sarafian 1986) shows that as the amount of mercury present increases, the amount of UMP is unaffected while UDP and UTP exhibit a mercury dose dependent decrease.
Due to the lack of available UTP, the RNA polymerase cannot add the uracil RNA nucleobase and mRNA transcription is inhibited (Alberts 2008). As a result, the patient suffers from a deficiency in cerebellar proteins (Sarafian 1986).
Excitotoxic Glutamate
A second factor that makes mercury particularly toxic to neurons in the cerebellum is the fact that cerebellar granule cells are glutamatergic (Clerici et al. 2004). Glutamate is an excitatory neurotransmitter. However, when too much is present, it has been shown to cause neural lesions (Castoldi 2001). Astrocytes normally take in the excess glutamate to prevent neurotoxic levels of it to accumulate (Alberts 2008).
However, when mercury is present, the astrocytes have a preference to take in the mercury in order to protect the cerebellar cells from exposure (Castoldi 2001). As the accumulation of mercury in the astrocytes increases, the capacity of the astrocytes to regulate the amount of extracellular glutamate present becomes limited (Castoldi 2001). Although the cerebellar cells are protected from the mercury, the concentration of glutamate can become unregulated to the point where the excitatory effects of the neurotransmitter can become toxic and cause neurodegeneration (Castoldi 2001).
Neuronal cell death occurs because the build up of excitatory glutamate and the overstimulation of its receptors lead to an increase in intracellular calcium levels (Castoldi 2001). It is suggested that the increase in calcium levels correlates with the activation of degrading enzymes, mitochondrial dysfunction, and cytoskeletal instability that can all contribute to neuronal death and result in lesions in the cerebellum (Castoldi 2001).
-Disease Etiology Section Completed by Ronald Alivia
A second factor that makes mercury particularly toxic to neurons in the cerebellum is the fact that cerebellar granule cells are glutamatergic (Clerici et al. 2004). Glutamate is an excitatory neurotransmitter. However, when too much is present, it has been shown to cause neural lesions (Castoldi 2001). Astrocytes normally take in the excess glutamate to prevent neurotoxic levels of it to accumulate (Alberts 2008).
However, when mercury is present, the astrocytes have a preference to take in the mercury in order to protect the cerebellar cells from exposure (Castoldi 2001). As the accumulation of mercury in the astrocytes increases, the capacity of the astrocytes to regulate the amount of extracellular glutamate present becomes limited (Castoldi 2001). Although the cerebellar cells are protected from the mercury, the concentration of glutamate can become unregulated to the point where the excitatory effects of the neurotransmitter can become toxic and cause neurodegeneration (Castoldi 2001).
Neuronal cell death occurs because the build up of excitatory glutamate and the overstimulation of its receptors lead to an increase in intracellular calcium levels (Castoldi 2001). It is suggested that the increase in calcium levels correlates with the activation of degrading enzymes, mitochondrial dysfunction, and cytoskeletal instability that can all contribute to neuronal death and result in lesions in the cerebellum (Castoldi 2001).
-Disease Etiology Section Completed by Ronald Alivia